This week in MathOnco 345
Darwinian evolution, digital twins, multiscale modeling, and antifragility
“This week in Mathematical Oncology” — August 14, 2025
> mathematical-oncology.org
From the editor:
Welcome to another edition of the Math Onco newsletter! Today’s edition contains papers on Darwinian evolution, digital twins, multiscale modeling, and one paper of my own, on antifragility!
Below, there is also an announcement for an interesting “EvoChat” virtual event, hosted by ISEEC.
Enjoy,
Jeffrey West
jeffrey.west@moffitt.org
The Approaching Darwinian Era in Oncology
Robert A. GatenbyCancer Systems Biology: Translational Mathematical Oncology (book)
Ravi Salgia, Mohit Kumar Jolly, Prakash Kulkarni, Govindan Rangarajanm
Multiscale information processing in the immune system
Navarro Quiroz R, Villarreal Camacho J, Zarate Peñata E, Bello Lemus Y, …, Rebolledo Cobos M, Fandiño Moreno J, Fiorillo-Moreno O, Navarro Quiroz ETowards a framework for predicting immunotherapy outcome: a hybrid multiscale mathematical model of immune response to vascular tumor growth
Sayyed Mohammad Ali Mortazavi & Bahar FiroozabadiIn silico Digital Twins of Bone Metastasis Enable Investigation of Tumor Progression and Therapy Response.
Marsilio L, Barrios S, Maksimovic S, Maccarini A, Serafini E, Grimaldi M, Heyman TJ, Cerveri P, Casarin S, Dondossola E.
Evolutionary antifragile therapy
Jeffrey West, Bina Desai, Maximilian Strobl, Jill Gallaher, Mark Robertson-Tessi, Andriy Marusyk, Alexander R. A. AndersonCalcium Signalling in Glioblastoma Networks of Different Topologies and Possible Treatments
Alexandra Shyntar, Thomas HillenInference of lineage hierarchies, growth and drug response mechanisms in cancer cell populations – without tracking
Andrea Piras, Federica Galvagno, Letizia Pizzini, Elena Grassi, Andrea Bertotti, Luca Primo, Antonio Celani, Alberto PuliafitoDifferentiation hierarchy in adult B cell acute lymphoblastic leukemia at clonal resolution
Alec Geßner, Adrien Jolly, Tjeerd P. Sijmonsma, Yves Matthess, Manuel Kaulich, Stefan Günther, Fabian Lang, Florian Buettner, Michael A. RiegerPolyclonal origins of human premalignant colorectal lesions
Debra Van Egeren, Ryan O. Schenck, Aziz Khan, Aaron M Horning, …, Zheng Hu, James M. Ford, Michael P. Snyder, Christina Curtis
Use this cell grammar to simplify systems biology modeling
The Mathematical Oncology Blog
Paul Macklin, Elana Fertig, Jeanette Johnson, Daniel Bergman, Genevieve Stein-O’Brien - September 12, 2025EvoChat hosted by ISEEC.
Title: How we define cancer matters
Speakers: Carlo Maley, Lucie Laplane, Joel Brown and Benjamin Spada
Date/Time: Oct 1st: 8am PST, 11am EST, 5pm CESTZoom Link: https://ucsb.zoom.us/j/83131113758?pwd=PV6vYTeP0tWR7IZ8CDb8PqAPBK9jpc.1
Abstract: Current definitions of cancer describe what cancer does but not what it is. Furthermore, they derive largely from human cases and may not apply to other multicellular organisms, especially those lacking a basal membrane. Comparative oncology, however, has shown that cancer occurs across a broad diversity of organisms. What definition should be adopted when framing cancer in a comparative and evolutionary perspective? And can an evolution-based definition be applied in clinical oncology? We will show that a universal definition of cancer not only helps provide a basis for progress in comparative oncology, but can also help to explain the diversity of cancer phenomena within humans.
Adam Palmer, (University of North Carolina)
Modeling tumor heterogeneity to understand the clinical efficacy of combination therapy
MCMC Seminar #2: Tumor heterogeneity is a central difficulty of cancer treatment and causes practically all cancer therapies to vary in effectiveness between patients. Combination therapy is a key strategy to overcome heterogeneity, but predicting which drug combinations will be effective has been a long unsolved problem. Because single drugs are variably effective, we have built models around the idea that combination therapy in human populations involves ‘variable effects plus variable effects’. I will describe studies that apply this principle to understand and to accurately predict the population-level activity of combination therapies in human clinical trials, including treatments with curative effect.
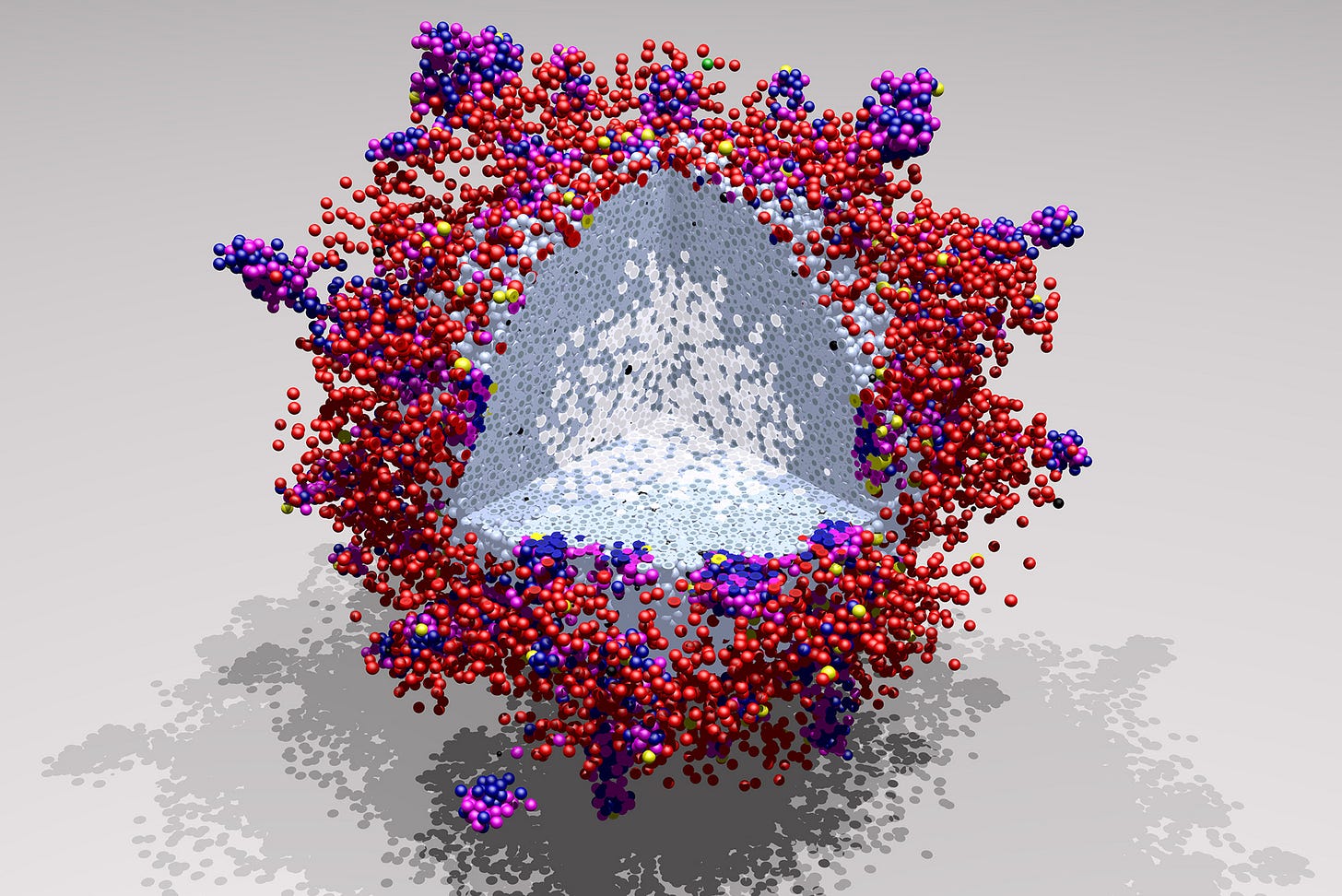
The newsletter now has a dedicated homepage where we post the cover artwork for each issue, curated by Maximilian Strobl, Sarah Groves, and Veronika Hofmann. We encourage submissions that coincide with the release of a recent paper from your group. This week’s artwork:
Based on the paper: Human interpretable grammar encodes multicellular systems biology models to democratize virtual cell laboratories published in Cell
Artist: Paul Macklin (@mathcancer.bsky.social) and Heber L Rocha (@heberlr.bsky.social)
Caption: 3D simulation of a tumor under attack by the immune system, constructed entirely with “plain language” cell behavior hypotheses that are automatically converted to simulation code. Cancer cells (pale blue) grow and divide but deplete oxygen, leading to hypoxia and necrotic death (white center). The cell death attracts macrophages (red) who switch to an inflammatory state (yellow) to guide cytotoxic T cells (blue and magenta) that attack the tumor. Macrophages and T cells can form temporary aggregates, and cell-cell communication is critical to successful tumor infiltration. Intuitively building and exploring the rules of cell behaviors allows scientists to tease out these interactions and visualize the dynamics of the evolving tumor ecosystem. In the article, we use these rules to create a “virtual laboratory” where teams can easily build models in plain human language without writing computer code, and explore how combining therapies can alter the rules to improve immune infiltration and tumor control. (See here for an animation).
Visit the mathematical oncology page to view jobs, meetings, and special issues. We will post new additions here, but the full list can found at mathematical-oncology.org.
1. Jobs
Current subscriber count: 2,369