This week in MathOnco 346
CAR-T, nanoparticles, mutation burden, drug-persisters, and antifragility...
“This week in Mathematical Oncology” — Oct 2, 2025
> mathematical-oncology.org
From the editor:
Today’s issue contains topics like modeling CAR-T, nanoparticles, mutation burden, drug-persisters, and more… I also wrote & published a blog post on our recent antifragility preprint.
Enjoy,
Jeffrey West
jeffrey.west@moffitt.org
CAR T-cell and oncolytic virus dynamics and determinants of combination therapy success for glioblastoma
Martina Conte, Agata Xella, Ryan T. Woodall, Kevin A. Cassady, Sergio Branciamore, Christine E. Brown, Russell C. RockneDrug-loaded nanoparticles for cancer therapy: a high-throughput multicellular agent-based modeling study
Yafei Wang, John Metzcar, Elmar Bucher, Heber Rocha, Vikram Jadhao, Randy Heiland, Hermann B. Frieboes, Paul MacklinNatalia L Komarova, Justin R Pritchard, Dominik Wodarz
Inheritable cell-states shape drug-persister correlations and population dynamics in cancer cells.
Iyer A, Alva A, Granada AE, Chakrabarti S.Mechanistic models position ceritinib as a nuclear integrity disrupting therapy in pediatric liver tumors.
Demir S, Kessler T, Hotes A, Häberle B, Hiyama E, Hishiki T, Indersie E, Branchereau S, Vokuhl C, Dorel M, Lehrach H, Lange B, Cairo S, Kappler R.Optimizing therapeutic outcomes with Mechanotherapy and Ultrasound Sonopermeation in solid tumors.
Koutsi M, Stylianopoulos T, Mpekris F.Modeling Propagation of Weakly Advantageous Mutations in Cancer Cells
Andrzej Polański, Mateusz Kania, Jarosław Gil, Wojciech Łabaj, Ewa Lach, Agnieszka SzcęsnaOrdinary differential equation model of cancer-associated fibroblast heterogeneity predicts treatment outcomes
Junho Lee & Eunjung Kim
The balance between intrinsic and ecological fitness reveals hidden regimes in eco-evolutionary population dynamics.
Barker-Clarke RJ, Gray JM, Leither S, Strobl MAR, Maltas J, Tadele DS, Hinczewski M, Scott JG.
Drug-induced proliferation curves preserve convexity
The Mathematical Oncology Blog
Jeffrey West: “Five years ago, we published a preprint titled “Antifragile therapy,” wherein we described the concept of antifragility, and how it might apply to cancer therapy. Antifragility intercepts conceptually with the idea of intermittent therapy, which introduces drug holidays to slow resistance evolution. We view this as a dose ‘perturbation’ where the timing of dose perturbations (or lack thereof, in terms of treatment holidays) can improve therapeutic effectiveness. So, why the long gap between the first manuscript in 2020 and this new preprint, in 2025?“Kit Curtius (UC San Diego)
Mathematical modeling of cancer evolution to optimize prevention strategies
MCMC Seminar #3: Cancer initiation and progression is an evolutionary process. Genetic and epigenetic alterations underpin phenotypes that drive natural selection at the cellular level in precancerous stages. However, predicting later cancer formation often requires more than counting mutations. Understanding the dynamics is critical to predict individual cancer risk in patients and intervene effectively. I will present mathematical methods that incorporate multiscale data types (from population-level incidence to patient-level genotypes) to help answer complex questions such as when to offer screening tests in certain populations.
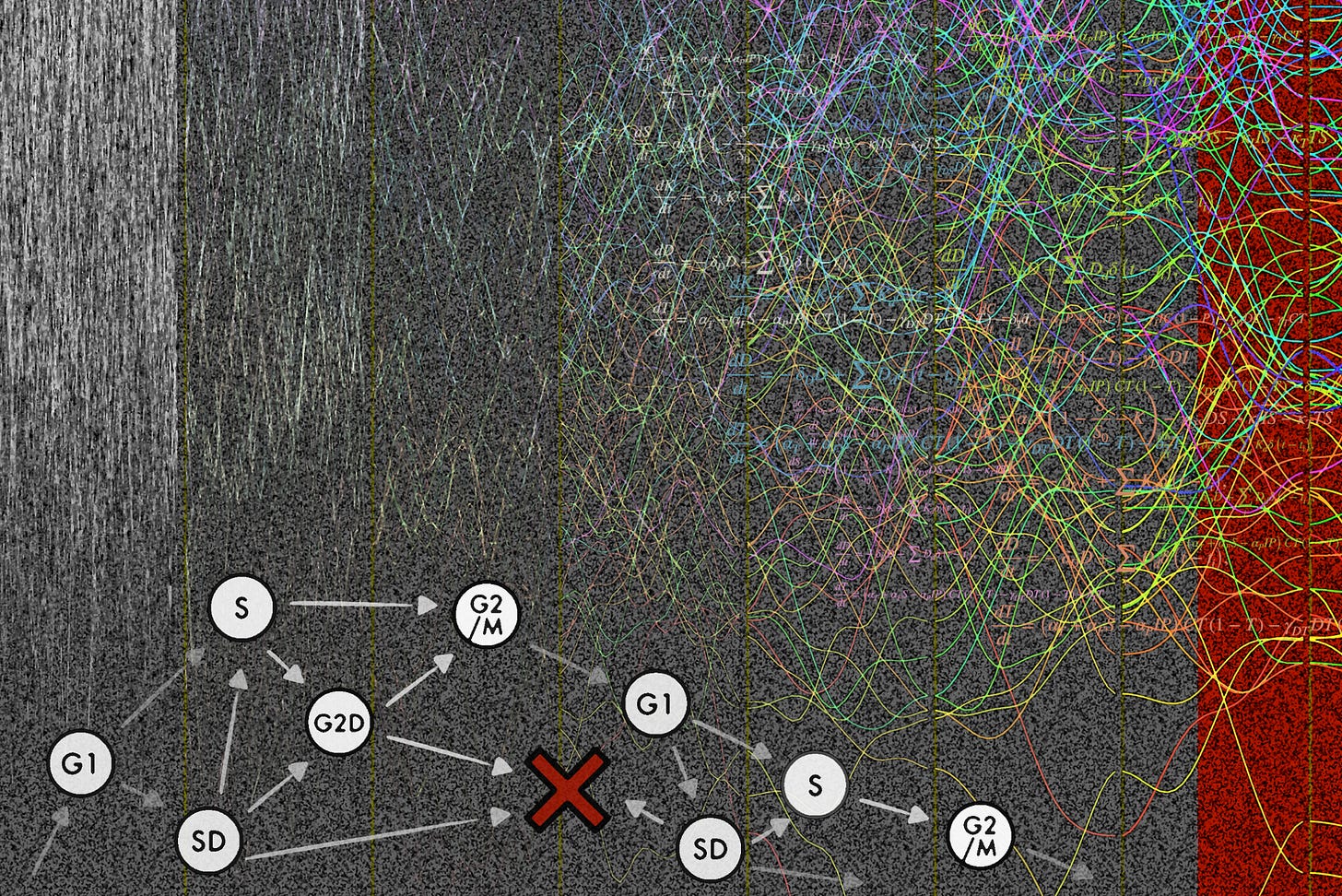
The newsletter now has a dedicated homepage where we post the cover artwork for each issue, curated by Maximilian Strobl, Sarah Groves, and Veronika Hofmann. We encourage submissions that coincide with the release of a recent paper from your group. This week’s artwork:
Based on the preprint: Controlling treatment toxicity in ovarian cancer to prime the patient for tumor extinction therapy published on bioRxiv
Artist: Maximilian Strobl (@StroblMAR), Kit Gallagher (@SciKit_G)
Caption: High-grade serous ovarian cancer (HGSOC) remains a challenging disease to treat due to high recurrence rates, acquired resistance, and cumulative toxicity. During the 12th IMO Workshop we explored the development of a “multi-strike, extinction protocol” for frontline treatment of HGSOC, which aims to eradicate tumors not through a singular “magic bullet” but through a series of different therapies (“strikes”). In developing such a protocol there are three key questions: what treatments to give, how long to give it, and when to switch? Illustrated here are how we integrated mathematical modeling and data analysis to address these challenges: i) we devised a method to identify patterns in fluctuating methylation sites to assess hematopoietic toxicity risk from liquid biopsy (colorful lines in the background), ii) we used mathematical cell cycle modeling to design effective low- dose combinations of targeted agents (model diagram in bottom left), and iii) we developed a new mathematical model to analyze the interplay between chemotherapy, gut microbiome toxicity, and immunotherapy, demonstrating how mitigating microbiome damage could enhance immune response (equations in top right). The next iteration of the IMO workshop is now only a month away! To get yourself into the mood, check out reports from previous workshops on the bioRxiv channel.
Visit the mathematical oncology page to view jobs, meetings, and special issues. We will post new additions here, but the full list can found at mathematical-oncology.org.
1. Jobs
Current subscriber count: 2,390